acid hydrolysis of esters is of reversible nature, while alkaline hydrolysis is irreversible . why ?
Dear student
Please find below the solution to the asked query:
Acidic hydrolysis is the reverse of esterification reaction. The ester is heated with a large excess of water containing a strong-acid catalyst. The reaction is reversible and does not go to completion.
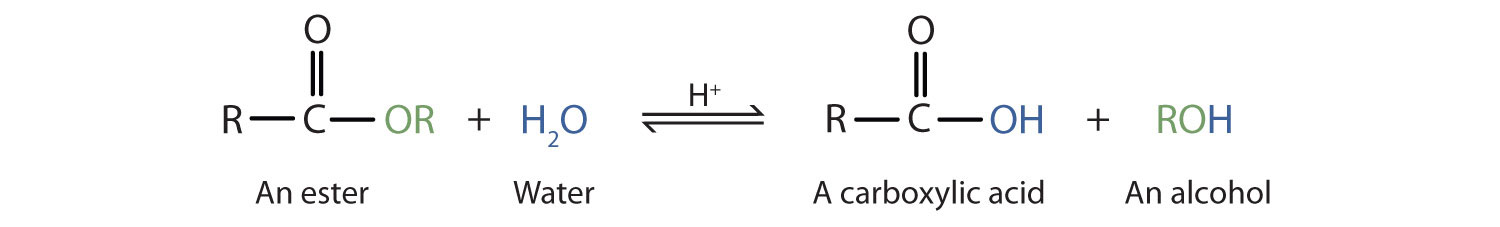
When a base (such as sodium hydroxide [NaOH] or potassium hydroxide [KOH]) is used to hydrolyze an ester, the products are a carboxylate salt and an alcohol. Because soaps are prepared by the alkaline hydrolysis of fats and oils, alkaline hydrolysis of esters is called saponification.
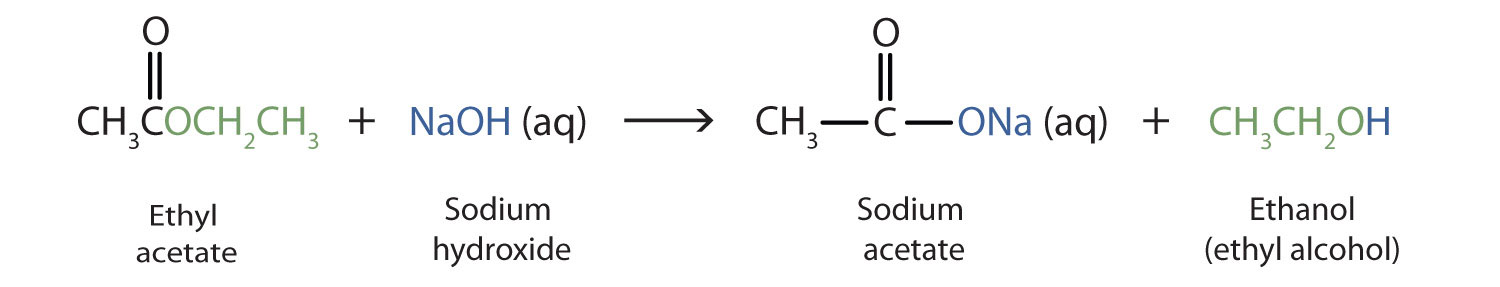
This reaction is reversible , because, the carboxylate ion (a nucleophile) which is formed, cannot attack alcohol (which is another nucleophile)
Hope this information will clear your doubts regarding the topic.
If you have any other doubts please ask here on the forum and our experts will try to solve them as soon as possible.
Regards

