Charge
- there are two kinds of charge, positive and negative
- like charges repel, unlike charges attract
- positive charge comes from having more protons than electrons; negative charge comes from having more electrons than protons
- charge is quantized, meaning that charge comes in integer multiples of the elementary charge e
- charge is conserved
Probably everyone is familiar with the first three concepts, but what does it mean for charge to be quantized? Charge comes in multiples of an indivisible unit of charge, represented by the letter e. In other words, charge comes in multiples of the charge on the electron or the proton. These things have the same size charge, but the sign is different. A proton has a charge of +e, while an electron has a charge of -e.
Electrons and protons are not the only things that carry charge. Other particles (positrons, for example) also carry charge in multiples of the electronic charge. Those are not going to be discussed, for the most part, in this course, however.
Putting "charge is quantized" in terms of an equation, we say:
q = n e
q is the symbol used to represent charge, while n is a positive or negative integer, and e is the electronic charge, 1.60 x 10-19 Coulombs.
The Law of Conservation of Charge
The Law of conservation of charge states that the net charge of an isolated system remains constant.
If a system starts out with an equal number of positive and negative charges, there¹s nothing we can do to create an excess of one kind of charge in that system unless we bring in charge from outside the system (or remove some charge from the system). Likewise, if something starts out with a certain net charge, say +100 e, it will always have +100 e unless it is allowed to interact with something external to it.
Charge can be created and destroyed, but only in positive-negative pairs.
Table of elementary particle masses and charges:
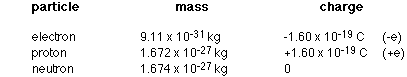
Electrostatic charging
Forces between two electrically-charged objects can be extremely large. Most things are electrically neutral; they have equal amounts of positive and negative charge. If this wasn¹t the case, the world we live in would be a much stranger place. We also have a lot of control over how things get charged. This is because we can choose the appropriate material to use in a given situation.
Metals are good conductors of electric charge, while plastics, wood, and rubber are not. They¹re called insulators. Charge does not flow nearly as easily through insulators as it does through conductors, which is why wires you plug into a wall socket are covered with a protective rubber coating. Charge flows along the wire, but not through the coating to you.
Materials are divided into three categories, depending on how easily they will allow charge (i.e., electrons) to flow along them. These are:
- conductors - metals, for example
- semi-conductors - silicon is a good example
- insulators - rubber, wood, plastic for example
Most materials are either conductors or insulators. The difference between them is that in conductors, the outermost electrons in the atoms are so loosely bound to their atoms that they¹re free to travel around. In insulators, on the other hand, the electrons are much more tightly bound to the atoms, and are not free to flow. Semi-conductors are a very useful intermediate class, not as conductive as metals but considerably more conductive than insulators. By adding certain impurities to semi-conductors in the appropriate concentrations the conductivity can be well-controlled.
There are three ways that objects can be given a net charge. These are:
- Charging by friction - this is useful for charging insulators. If you rub one material with another (say, a plastic ruler with a piece of paper towel), electrons have a tendency to be transferred from one material to the other. For example, rubbing glass with silk or saran wrap generally leaves the glass with a positive charge; rubbing PVC rod with fur generally gives the rod a negative charge.
- Charging by conduction - useful for charging metals and other conductors. If a charged object touches a conductor, some charge will be transferred between the object and the conductor, charging the conductor with the same sign as the charge on the object.
- Charging by induction - also useful for charging metals and other conductors. Again, a charged object is used, but this time it is only brought close to the conductor, and does not touch it. If the conductor is connected to ground (ground is basically anything neutral that can give up electrons to, or take electrons from, an object), electrons will either flow on to it or away from it. When the ground connection is removed , the conductor will have a charge opposite in sign to that of the charged object.
An example of induction using a negatively charged object and an initially-uncharged conductor (for example, a metal ball on a plastic handle).
(1) bring the negatively-charged object close to, but not touching, the conductor. Electrons on the conductor will be repelled from the area nearest the charged object.
(2) connect the conductor to ground. The electrons on the conductor want to get as far away from the negatively-charged object as possible, so some of them flow to ground.
(3) remove the ground connection. This leaves the conductor with a deficit of electrons.
(4) remove the charged object. The conductor is now positively charged.
A practical application involving the transfer of charge is in how laser printers.

