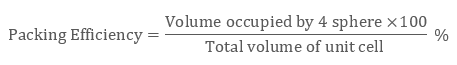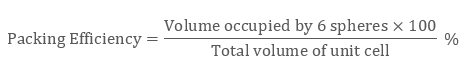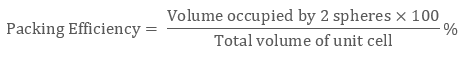Among bcc hcp and ccp which is more efficient and why
Dear student,
The packing efficient of HCP and CCP are same and more efficient than BCC.
HCP and CCP Structures
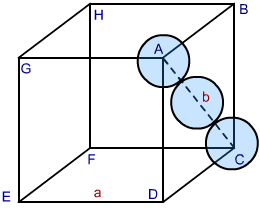
In both types of packing hexagonal closed packing or cubic closed packing the packing efficiency is the same. In order to calculate packing efficiency in a cubic closed structure, we consider a unit cell with edge length “a” and face diagonal AC to be “b”.
Image 2: CCP Structure
Looking at the ABCD face of the cube, we see a triangle ABC. Let the radius of the each sphere ball be ‘r’. Correlating radius and edge of the cube, we proceed as follows:
In △ ABC, by Pythagoras theorem we can write:
AC2 = BC2 + AB2
Since AC = b and BC = AB = a, substituting the values in the above relation we get
b2 = a2 + a2
b2 = 2a2
b = √2 a….. (1)
also edge b in terms of radius ‘r’ equals
b = 4r … (2)
From (1) and (2) we can write
4r = √2 a
or
a = 2 √2 r
As there are 4 spheres in a ccp structure unit cell, the total volume occupied by them will be
4 × 4 / 3 π r3
Also, total volume of a cube is (edge length)3 that is, ( a3) or in terms of r it is (2 √2 r)3, therefore packing efficiency will be:
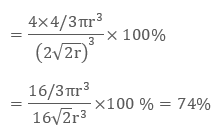
Therefore packing efficiency in fcc and hcp structures is 74%
Similarly in HCP lattice the relation between radius ‘r’ and edge length of unit cell “a” is r = 2a and number of atoms is 6.
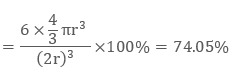
Body-Centred Cubic Structures
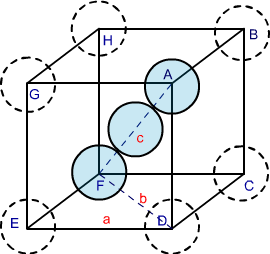
In body-centred cubic structures, the three atoms are diagonally arranged. To find the packing efficiency we consider a cube with edge length a, face diagonal length b and cube diagonal as c.
Image 3: BCC structure
In △ EFD according to Pythagoras theorem
b2 = a2 + a2
b2 = 2a2
b = √2 a
Now in △ AFD according to Pythagoras theorem
c2 = a2 + b2 = a2 + 2a2
c2 = 3a2
c = √3 a
If the radius of each sphere is ‘r’ then we can write
c = 4r
√3 a = 4r
r = √3/ 4 a
As there are two atoms in the bcc structure the volume of constituent spheres will be
2 × (4/3) π r3

Therefore packing efficiency of the body-centred unit cell is 68%.
Regards.