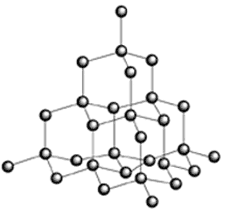
Diamonds has a high melting and boiling point. But it forms covalent bonds. And a covalent compound has low melting and boiling point. How is this possible?
No Diamonds had 3D structure It is just an allotrope of Carbon and Carbon forms Compounds(and not Allotropes) by Covalent Bonding.