A solution is a homogeneous mixture of one or more solutes dissolved in a solvent.
- solvent: the substance in which a solute dissolves to produce a homogeneous mixture
- solute: the substance that dissolves in a solvent to produce a homogeneous mixture
Note that the solvent is the substance that is present in the greatest amount.
Many different kinds of solutions exist. For example, a solute can be a gas, a liquid or a solid. Solvents can also be gases, liquids or solids.
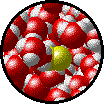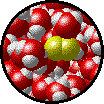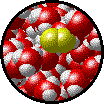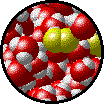
The following figures show the microscopic behavior of several different kinds of solutions. Note that in each case, the solute particles are uniformly distributed among the solvent particles.
 |  |
| Microscopic view of Br2 gas (solute) dissolved in Ar gas (solvent). | Microscopic view of Ar gas (solute) dissolved in liquid H2O (solvent). |
 |  |
| Microscopic view of Br2liquid (solute) dissolved in liquid H2O (solvent). | Microscopic view of solid NaCl (solute) dissolved in liquid H2O (solvent).Note that the ionic solid, NaCl, produces Na+ ions (blue) and Cl- ions (green) when dissolved in water. |
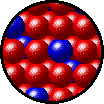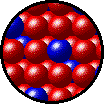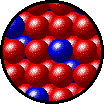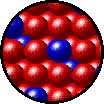 |
| Microscopic view of solid Kr (solute, blue) dissolved in solid Xe (solvent, red). |






