Which is more stable: [Co(OX)3]3- or [CoF6]3- ? Why?
Dear student
Please find the solution to the asked query:
[Co(ox)3]3- is more stable than [CoF6]3- because as oxalate is a bidentate ligand it forms a chelated ring compound, by occupying all the coordination sites, which is more stable than the normal compound containing a monodentate ligand like F-
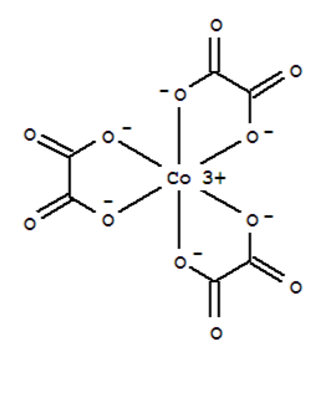

Hope this information will clear your doubts regarding the topic. If you have any other doubts please ask here on the forum and our experts will try to solve them as soon as possible.
Regards
Please find the solution to the asked query:
[Co(ox)3]3- is more stable than [CoF6]3- because as oxalate is a bidentate ligand it forms a chelated ring compound, by occupying all the coordination sites, which is more stable than the normal compound containing a monodentate ligand like F-


Hope this information will clear your doubts regarding the topic. If you have any other doubts please ask here on the forum and our experts will try to solve them as soon as possible.
Regards

